Sinovac Biotech Ltd. (SINOVAC) is a China-based biopharmaceutical company that focuses on the R&D, manufacturing, and commercialization of vaccines that protect against human infectious diseases.
SINOVAC's product portfolio includes vaccines against COVID-19, enterovirus 71 (EV71) infected Hand-Foot-Mouth disease (HFMD), hepatitis A, varicella, influenza, poliomyelitis, pneumococcal disease, and mumps.
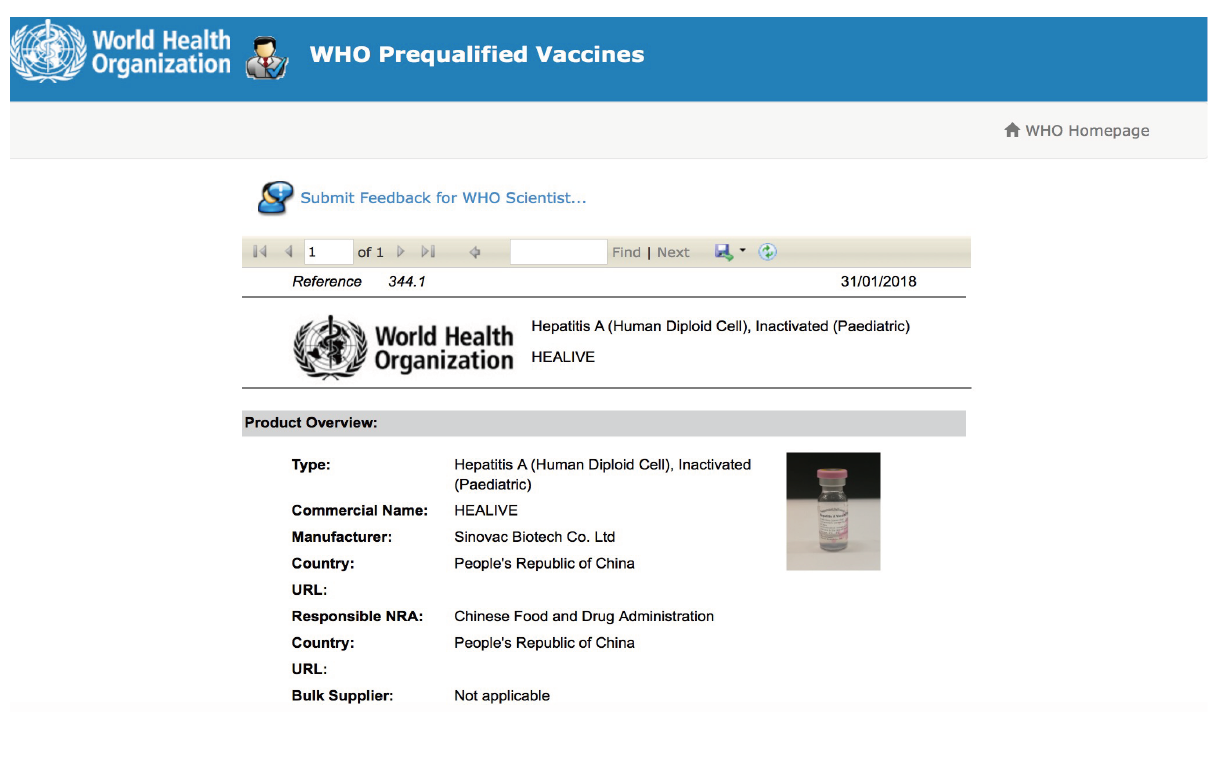
The COVID-19 vaccine, CoronaVac®, has been approved for use in more than 60 countries and regions worldwide. The hepatitis A vaccine, Healive®, passed WHO prequalification requirements in 2017. The EV71 vaccine, Inlive®, is an innovative vaccine under "Category 1 Preventative Biological Products" and was commercialized in China in 2016. In 2022, SINOVAC's Sabin-strain inactivated polio vaccine (sIPV) and varicella vaccine were prequalified by the WHO.
SINOVAC was the first company to be granted approval for its H1N1 influenza vaccine Panflu.1®, which has supplied the Chinese government's vaccination campaign and stockpiling program. The Company is also the only supplier of the H5N1 pandemic influenza vaccine, Panflu®, to the Chinese government stockpiling program.
SINOVAC continually dedicates itself to new vaccine R&D, with more combination vaccine products in its pipeline, and constantly explores global market opportunities. SINOVAC plans to conduct more extensive and in-depth trade and cooperation with additional countries, and business and industry organizations.
MissionSupply vaccines to eliminate human diseases.
 EN
EN